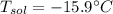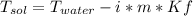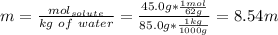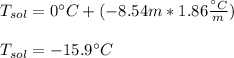Chemistry, 24.07.2020 21:01 acornelas7128
What is the freezing point of a solution prepared from 45.0 g ethylene glycol (C2H6O2) and 85.0 g H2O? Kf of water is 1.86°C/m.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1


Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
What is the freezing point of a solution prepared from 45.0 g ethylene glycol (C2H6O2) and 85.0 g H2...
Questions


Social Studies, 22.09.2021 22:50

Geography, 22.09.2021 22:50


English, 22.09.2021 22:50




Mathematics, 22.09.2021 22:50


Biology, 22.09.2021 22:50



Biology, 22.09.2021 22:50

History, 22.09.2021 22:50




Social Studies, 22.09.2021 22:50