

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1
You know the right answer?
How many moles of ZnCl2 will be produced from 61.0 g of Zn, assuming HCl is excess?...
Questions

Chemistry, 13.09.2020 20:01

Mathematics, 13.09.2020 20:01

Mathematics, 13.09.2020 20:01

Mathematics, 13.09.2020 20:01

Mathematics, 13.09.2020 20:01

Mathematics, 13.09.2020 20:01

Mathematics, 13.09.2020 20:01

English, 13.09.2020 20:01

Mathematics, 13.09.2020 20:01

Social Studies, 13.09.2020 20:01

English, 13.09.2020 20:01

Mathematics, 13.09.2020 20:01

Mathematics, 13.09.2020 20:01

Mathematics, 13.09.2020 20:01

Spanish, 13.09.2020 20:01

Mathematics, 13.09.2020 20:01

Mathematics, 13.09.2020 20:01

Mathematics, 13.09.2020 20:01

Mathematics, 13.09.2020 20:01

Mathematics, 13.09.2020 20:01

 will be produced.
will be produced.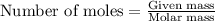

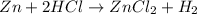
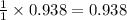 moles of
moles of 

