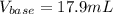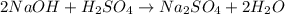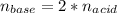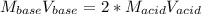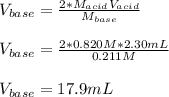Chemistry, 23.07.2020 21:01 shongmadi77
Calculate the volume, in milliliters, of a 0.211 M solution of NaOH that will completely neutralize each of the following. 2.30 mL of a 0.820 M solution of H2SO4. Express the volume in milliliters to three significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Imagine that you own a property that is exactly 2.2 acres large. you want to sell your property, but your realtor tells you that you cannot sell your land by the acre. in order to sell your land you need to determine the area you own in units of square meters? given that there are 1.6 kilometers in 1 mile and 640 acres in 1 square mile, what is the area of land that you own in square meters square meters?
Answers: 2

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 08:30
Since the gas in your graduated cylinder is a mixture of butane and water vapor, you must determine the partial pressure of the butane, pbutane, alone. to do this, consult a reference and record the partial pressure of the water vapor, pwater, at the temperature you recorded. use the following formula to compute the partial pressure of the butane. pbutane = atmosphere - pwater use the following combined gas law formula and compute the volume that the butane sample will occupy at stp. (hint: convert both temperatures to kelvin.) pbutane x voriginal = pstandard x vfinal troom tstandard use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. grams of butane you used “x” grams of butane ml of butane corrected to stp = 22,400 ml compute the theoretical molar mass of butane based on its formula and the atomic masses on the periodic table. compare your experimental results from #3 to the theoretical value of #4, computing a percent error of your findings using this formula: % error = measured value - accepted value x 100 accepted value use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. need asap
Answers: 1

Chemistry, 23.06.2019 06:30
Acompound has the molecular formula c3h8. which class of organic compounds does it belong to?
Answers: 2
You know the right answer?
Calculate the volume, in milliliters, of a 0.211 M solution of NaOH that will completely neutralize...
Questions

Biology, 20.10.2020 04:01

Mathematics, 20.10.2020 04:01

Spanish, 20.10.2020 04:01

Mathematics, 20.10.2020 04:01


Mathematics, 20.10.2020 04:01

English, 20.10.2020 04:01

Social Studies, 20.10.2020 04:01


Chemistry, 20.10.2020 04:01

Mathematics, 20.10.2020 04:01


Mathematics, 20.10.2020 04:01


English, 20.10.2020 04:01

Social Studies, 20.10.2020 04:01


Mathematics, 20.10.2020 04:01