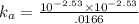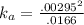Chemistry, 23.07.2020 20:01 brendancrow5927
Enough of a monoprotic weak acid is dissolved in water to produce a 0.01660.0166 M solution. The pH of the resulting solution is 2.532.53 . Calculate the Ka for the acid.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 23:00
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2
You know the right answer?
Enough of a monoprotic weak acid is dissolved in water to produce a 0.01660.0166 M solution. The pH...
Questions



Chemistry, 30.03.2021 21:00

Mathematics, 30.03.2021 21:00

Mathematics, 30.03.2021 21:00


Mathematics, 30.03.2021 21:00

Mathematics, 30.03.2021 21:00

Physics, 30.03.2021 21:00

Chemistry, 30.03.2021 21:00

Geography, 30.03.2021 21:00


History, 30.03.2021 21:00

Mathematics, 30.03.2021 21:00

Mathematics, 30.03.2021 21:00


Spanish, 30.03.2021 21:00

History, 30.03.2021 21:00

Mathematics, 30.03.2021 21:00

![[ H^+]=10^{-2.53}](/tpl/images/0711/9511/3a08a.png)
![[ X^-]=10^{-2.53}](/tpl/images/0711/9511/d1b85.png)