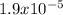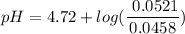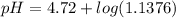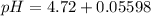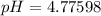Chemistry, 23.07.2020 19:01 moneyyfletcher
A 25.0-mL sample of 0.150 M hydrazoic acid, HN3, is titrated with a 0.150 M NaOH solution. What is the pH after 13.3 mL of base is added? The Ka of hydrazoic acid = 1.9 x 10-5.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1
You know the right answer?
A 25.0-mL sample of 0.150 M hydrazoic acid, HN3, is titrated with a 0.150 M NaOH solution. What is t...
Questions

Social Studies, 29.11.2021 04:20


Health, 29.11.2021 04:20



Mathematics, 29.11.2021 04:20

Mathematics, 29.11.2021 04:20

Mathematics, 29.11.2021 04:20

History, 29.11.2021 04:20

Mathematics, 29.11.2021 04:20


Mathematics, 29.11.2021 04:20



Mathematics, 29.11.2021 04:20

History, 29.11.2021 04:20


Mathematics, 29.11.2021 04:20


Mathematics, 29.11.2021 04:20


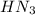 =
= 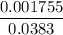 = 0.0458 M
= 0.0458 M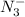 =
= 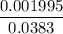 = 0.0521 M
= 0.0521 M