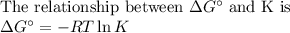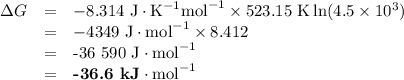Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2


Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

You know the right answer?
the equilibrium constant at 250C for the equation PCl5 PCl3 is kp 4.5 x 10^3 bar calculate value of...
Questions

Mathematics, 02.12.2020 06:20

Geography, 02.12.2020 06:20


Mathematics, 02.12.2020 06:20


Mathematics, 02.12.2020 06:20

History, 02.12.2020 06:20

Chemistry, 02.12.2020 06:20

English, 02.12.2020 06:20



History, 02.12.2020 06:20


Medicine, 02.12.2020 06:20

Mathematics, 02.12.2020 06:20

Computers and Technology, 02.12.2020 06:20

Mathematics, 02.12.2020 06:20

Mathematics, 02.12.2020 06:20

Mathematics, 02.12.2020 06:20

History, 02.12.2020 06:20