
Chemistry, 23.07.2020 05:01 kaylaunderwood470
The pH of a solution prepared by mixing 40.00 mL of 0.10 M NH3 with 50.00 mL of 0.10 M NH4Cl and 30mL of 0.05 M H2SO4 is 5.17. Assume that the volume of the solutions are additive . What would be the Ka for NH4

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 23.06.2019 04:31
Pls i will do pls imma diewhat forms white light? (4 points)a. combination of all wavelengths of ultraviolet light b. combination of all wavelengths of visible lightc. absorption of electromagnetic waves d. absorption of infrared rays
Answers: 2

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1
You know the right answer?
The pH of a solution prepared by mixing 40.00 mL of 0.10 M NH3 with 50.00 mL of 0.10 M NH4Cl and 30m...
Questions

Mathematics, 05.09.2019 06:10







Mathematics, 05.09.2019 06:10


Mathematics, 05.09.2019 06:10

Social Studies, 05.09.2019 06:10


Computers and Technology, 05.09.2019 06:10

Mathematics, 05.09.2019 06:10



Mathematics, 05.09.2019 06:10



Mathematics, 05.09.2019 06:10

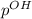 =14-56.17
=14-56.17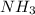 = 40.00 ml
= 40.00 ml 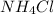 = 50.00 ml
= 50.00 ml
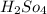 = 30 ml
= 30 ml
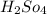 =0.05 M
=0.05 M
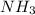 =
= 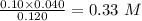
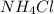 =
= 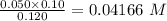 calculating the new concentrated value of
calculating the new concentrated value of 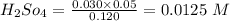 when 1 mol
when 1 mol 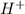 so, 0.0125 in
so, 0.0125 in 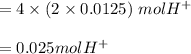
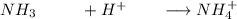
![P^{H}= p^{kb}|+ \log\frac{[NH_4^{+}]}{[NH_3]}\\\\8.83=p^{kb}+\log\frac{0.0667}{8.3 \times 10^{-3}}\\\\p^{kb}=8.83-0.9069\\\\ \ \ \ =7.7231 \\\\\ The P^{kb} \ for \ NH_3 \ is =7.7231\\\\\ The P^{kb} \ for N^{+}H_4=14-7.7231\\\\\ \ \ \ \ \ =6.2769](/tpl/images/0711/6580/727d8.png)


