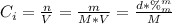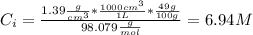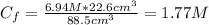Chemistry, 23.07.2020 03:01 hncriciacheichi
A solution of 49.0% H2SO4 by mass has a density of 1.39 g cm−3 at 293 K. A 22.6 cm3 sample of this solution is mixed with enough water to increase the volume of the solution to 88.5 cm3 . Find the molarity of sulfuric acid in this solution.

Answers: 1


Another question on Chemistry




Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2
You know the right answer?
A solution of 49.0% H2SO4 by mass has a density of 1.39 g cm−3 at 293 K. A 22.6 cm3 sample of this s...
Questions


Social Studies, 31.08.2021 14:20

Chemistry, 31.08.2021 14:20

Engineering, 31.08.2021 14:20

Mathematics, 31.08.2021 14:20


English, 31.08.2021 14:20



English, 31.08.2021 14:20

World Languages, 31.08.2021 14:20

Mathematics, 31.08.2021 14:20


Social Studies, 31.08.2021 14:20

Computers and Technology, 31.08.2021 14:20

Mathematics, 31.08.2021 14:20


English, 31.08.2021 14:20

Mathematics, 31.08.2021 14:20

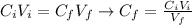
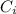 : is the initial concentration of the acid
: is the initial concentration of the acid 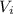 : is the initial volume of the solution = 22.6 cm³
: is the initial volume of the solution = 22.6 cm³ 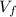 : is the final volume of the solution = 88.5 cm³
: is the final volume of the solution = 88.5 cm³