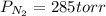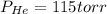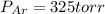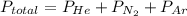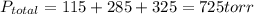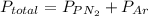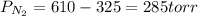A 3.00 L cylinder at 25 degrees Celsius contains a mixture of 3 gases: He, N2, and Ar at partial pressures of 115, 285, and 325 torr, respectively. If all the He is removed from the mixture and the temperature does not change, what will be the partial pressure, in torr, of the N2

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2

Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3
You know the right answer?
A 3.00 L cylinder at 25 degrees Celsius contains a mixture of 3 gases: He, N2, and Ar at partial pre...
Questions

Mathematics, 16.02.2020 08:02




Physics, 16.02.2020 08:06

Mathematics, 16.02.2020 08:06


Mathematics, 16.02.2020 08:12

Advanced Placement (AP), 16.02.2020 08:13


Mathematics, 16.02.2020 08:17

Mathematics, 16.02.2020 08:20


Biology, 16.02.2020 08:27


English, 16.02.2020 08:28


History, 16.02.2020 08:29