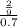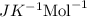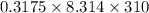Chemistry, 22.07.2020 23:01 MidnightAIY179
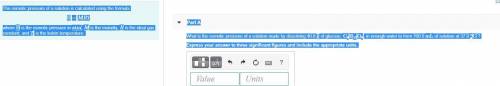
The osmotic pressure of a solution is calculated using the formula Π=MRT where Π is the osmotic pressure in atm, M is the molarity, R is the ideal gas constant, and T is the kelvin temperature. Part A What is the osmotic pressure of a solution made by dissolving 40.0 g of glucose, C6H12O6, in enough water to form 700.0 mL of solution at 37.0 ∘C ? Express your answer to three significant figures and include the appropriate units. nothing nothing

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2
You know the right answer?
The osmotic pressure of a solution is calculated using the formula Π=MRT where Π is the osmotic pres...
Questions

English, 27.09.2021 19:20

Business, 27.09.2021 19:20




Mathematics, 27.09.2021 19:20


Mathematics, 27.09.2021 19:30

Social Studies, 27.09.2021 19:30

Mathematics, 27.09.2021 19:30



Chemistry, 27.09.2021 19:30


Computers and Technology, 27.09.2021 19:30

Mathematics, 27.09.2021 19:30




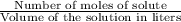
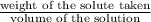
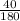
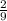 moles
moles