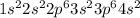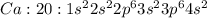Chemistry, 22.07.2020 20:01 yselahernandez02
where 1s, 2s, and 2p are the occupied subshells, and the superscript "2" is the number of electrons in each of these subshells. Use the rules for determining electron configurations to write the electron configuration for Ca. Express your answer in complete form in order of orbital filling. For example, 1s22s2 should be entered as 1s^22s^2.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:40
Which of the following is a testable hypothesis? a. if i brush my teeth, i will get fewer cavities than if i don't brush my teeth. b. green toothpaste tastes better than blue toothpaste or red toothpaste. c. smart, careful, healthy people always brush their teeth. d. it's wrong to not brush your teeth before you have an important conversation with someone.
Answers: 1

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
You know the right answer?
where 1s, 2s, and 2p are the occupied subshells, and the superscript "2" is the number of electrons...
Questions


Mathematics, 21.07.2019 12:40


Mathematics, 21.07.2019 12:40

Mathematics, 21.07.2019 12:40

Physics, 21.07.2019 12:40

Mathematics, 21.07.2019 12:40

Mathematics, 21.07.2019 12:40




Biology, 21.07.2019 12:40



Arts, 21.07.2019 12:40



Business, 21.07.2019 12:40


Mathematics, 21.07.2019 12:40