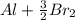Chemistry, 21.07.2020 18:01 darknessmidnight207
Which one of the following reactions must be carried out in an electrolytic cell rather than in an electrochemical cell?
a. Zn2+ + Ca → Zn + Ca2+
b. Al3+ + 3Br– → Al + (3/2)Br2
c. 2Al + 3Fe2+ → 2Al3+ + 3Fe
d. H2 + I2(s) → 2H+ + 2I–

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2
You know the right answer?
Which one of the following reactions must be carried out in an electrolytic cell rather than in an e...
Questions


History, 23.09.2021 16:10



English, 23.09.2021 16:10

Computers and Technology, 23.09.2021 16:10



Physics, 23.09.2021 16:10


Computers and Technology, 23.09.2021 16:10

English, 23.09.2021 16:10

Mathematics, 23.09.2021 16:10


English, 23.09.2021 16:10

Social Studies, 23.09.2021 16:10

Mathematics, 23.09.2021 16:10

Health, 23.09.2021 16:10

Mathematics, 23.09.2021 16:10

Social Studies, 23.09.2021 16:20

 →
→