Chemistry, 19.07.2020 01:01 dontcareanyonemo
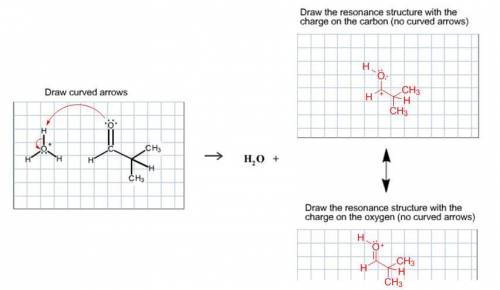
A proton transfer reaction can occur when a carbonyl compound is placed in an aqueous acidic solution. Water and a charged conjugate acid are produced. The resulting organic compound is resonance stabilized. Draw the curved arrows for the proton transfer and draw both resonance-stabilized organic products in the appropriate boxes. Be sure to include all lone pairs and nonzero formal charges. Do not draw the water product.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1


Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
You know the right answer?
A proton transfer reaction can occur when a carbonyl compound is placed in an aqueous acidic solutio...
Questions

Business, 30.01.2020 19:03

Advanced Placement (AP), 30.01.2020 19:03


Biology, 30.01.2020 19:03

Mathematics, 30.01.2020 19:03

Chemistry, 30.01.2020 19:03

Physics, 30.01.2020 19:03

Chemistry, 30.01.2020 19:03

Mathematics, 30.01.2020 19:03




Physics, 30.01.2020 19:03


Business, 30.01.2020 19:03

Mathematics, 30.01.2020 19:03


Mathematics, 30.01.2020 19:03


History, 30.01.2020 19:03