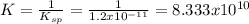Chemistry, 20.07.2020 01:01 littledudefromacross
If 75.0 mL of a 2.63 · 10-3 M NaOH is mixed with 125.0 mL of 1.80 · 10-3 M MgCl2, then calculate the reaction quotient and state if a precipitate will form? The Ksp of the expected precipitate is 1.2 · 10-11.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3
You know the right answer?
If 75.0 mL of a 2.63 · 10-3 M NaOH is mixed with 125.0 mL of 1.80 · 10-3 M MgCl2, then calculate the...
Questions

Chemistry, 30.10.2019 21:31

German, 30.10.2019 21:31


Health, 30.10.2019 21:31

Mathematics, 30.10.2019 21:31


Mathematics, 30.10.2019 21:31

Biology, 30.10.2019 21:31






History, 30.10.2019 21:31

Mathematics, 30.10.2019 21:31

Mathematics, 30.10.2019 21:31

History, 30.10.2019 21:31


Physics, 30.10.2019 21:31

Mathematics, 30.10.2019 21:31

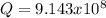
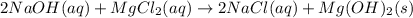
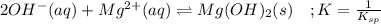
![\frac{1}{K_{sp}}=\frac{1}{[OH^-]^2[Mg^{2+}]}](/tpl/images/0709/8931/c7f27.png)
![[OH^-]=\frac{75.0mL*2.63x10^{-3}M}{75.0mL+125.0mL}=9.86x10^{-4}M](/tpl/images/0709/8931/2f439.png)
![[Mg^{2+}]=\frac{125.0mL*1.80x10^{-3}M}{75.0mL+125.0mL}=1.125x10^{-3}M](/tpl/images/0709/8931/0caa8.png)
![Q=\frac{1}{[OH^-]^2[Mg^{2+}]}=\frac{1}{(9.86x10^{-4})^2*1.125x10^{-3}} =9.143x10^8](/tpl/images/0709/8931/2e242.png)