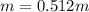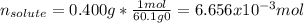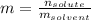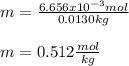Chemistry, 20.07.2020 01:01 savannahvargas512
3. A student adds 0.400g of n-propanol to 13.0 g of t-butanol. What is the molality of the solution? Show your calculations. (3 pts

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3


Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2
You know the right answer?
3. A student adds 0.400g of n-propanol to 13.0 g of t-butanol. What is the molality of the solution?...
Questions



Chemistry, 31.05.2021 07:50

English, 31.05.2021 07:50

Mathematics, 31.05.2021 07:50


Mathematics, 31.05.2021 07:50

Mathematics, 31.05.2021 07:50


Mathematics, 31.05.2021 07:50


Biology, 31.05.2021 07:50

Social Studies, 31.05.2021 07:50

Mathematics, 31.05.2021 07:50

Arts, 31.05.2021 07:50



Mathematics, 31.05.2021 07:50

English, 31.05.2021 07:50

Social Studies, 31.05.2021 07:50