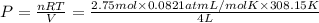Chemistry, 01.09.2019 10:00 familygrahambusiness
Assuming ideal behavior, how much pressure will 2.75 mol hydrogen gas exert on the walls of a 4.00 l container at 35.0ºc?
a. 17.4 atm b. 19.8 atm c. 278 atm d. 296 atm

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
Assuming ideal behavior, how much pressure will 2.75 mol hydrogen gas exert on the walls of a 4.00 l...
Questions

Spanish, 04.02.2021 21:50




Mathematics, 04.02.2021 21:50

History, 04.02.2021 21:50


Mathematics, 04.02.2021 21:50

Physics, 04.02.2021 21:50

Mathematics, 04.02.2021 21:50



Mathematics, 04.02.2021 21:50



Mathematics, 04.02.2021 21:50


Spanish, 04.02.2021 21:50

Mathematics, 04.02.2021 21:50

Mathematics, 04.02.2021 21:50