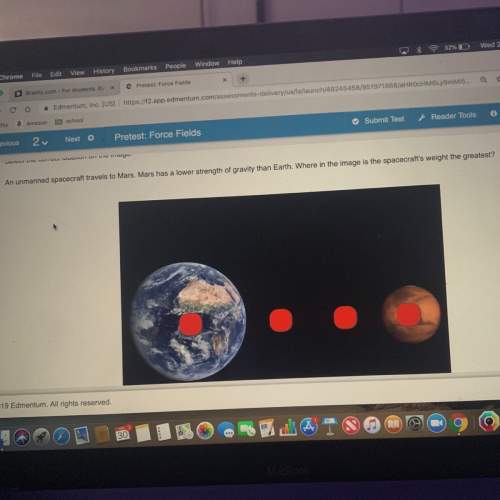
Chemistry, 18.07.2020 18:01 nyceastcoast
A metal has atoms with a radius of 162 pm. It crystallizes in a simple cubic unit cell. It has a density of 8.991 g/cm3. Calculate the molar mass of the metal in g/mol. Do not include units in your answer. Report your answer to the tenths place.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3
You know the right answer?
A metal has atoms with a radius of 162 pm. It crystallizes in a simple cubic unit cell. It has a den...
Questions







Mathematics, 19.01.2020 04:31

History, 19.01.2020 04:31

History, 19.01.2020 04:31


Social Studies, 19.01.2020 04:31


Chemistry, 19.01.2020 04:31

Mathematics, 19.01.2020 04:31


English, 19.01.2020 04:31

Health, 19.01.2020 04:31


History, 19.01.2020 04:31