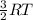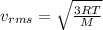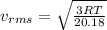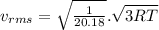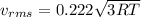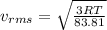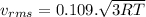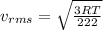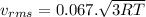Chemistry, 18.07.2020 04:01 Knownothing
A flask contains a mixture of neon Ne, krypton Kr, and radon Rn gases. (Hint: The molar mass of the is Ne 20.180 g/mol, of the Kr is 83.80g/mol, and of the Kr 222g/mol)
(A) Compare the average kinetic energies of the Ne and Kr.
(B) Comparethe average kinetic energies of the Kr and Rn.
(C) Compare the average kinetic energies of the Rn and Ne.
(D) Compare the root-mean-square speeds of the Ne and Kr.
(E) Compare the root-mean-square speeds of the Kr and Rn.
(F) Compare the root-mean-square speeds of the Rn and Ne.

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1

Chemistry, 23.06.2019 08:30
What percentage of energy used in the u.s is produced from fossil fuels
Answers: 2
You know the right answer?
A flask contains a mixture of neon Ne, krypton Kr, and radon Rn gases. (Hint: The molar mass of the...
Questions




Mathematics, 18.03.2021 03:00

Biology, 18.03.2021 03:00


Social Studies, 18.03.2021 03:00

Biology, 18.03.2021 03:00



Biology, 18.03.2021 03:00

Geography, 18.03.2021 03:00


Mathematics, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00

History, 18.03.2021 03:00