Chemistry, 17.07.2020 01:01 hiddenauthors436
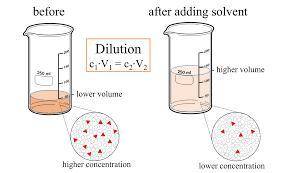
A volumetric flask contains 25.0 mL of a 14% m/V sugar solution. If 2.5 mL of this solution is added to 22.5 mL of distilled water, what is the % m/V of the new solution.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1
You know the right answer?
A volumetric flask contains 25.0 mL of a 14% m/V sugar solution. If 2.5 mL of this solution is added...
Questions


Mathematics, 27.08.2021 07:20

Mathematics, 27.08.2021 07:20

Mathematics, 27.08.2021 07:20

Mathematics, 27.08.2021 07:20

Mathematics, 27.08.2021 07:20

Chemistry, 27.08.2021 07:20

Mathematics, 27.08.2021 07:20


Computers and Technology, 27.08.2021 07:20

Mathematics, 27.08.2021 07:20



World Languages, 27.08.2021 07:20

Business, 27.08.2021 07:20


Mathematics, 27.08.2021 07:20

Mathematics, 27.08.2021 07:20