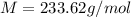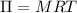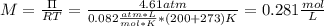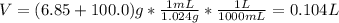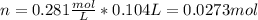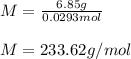Chemistry, 17.07.2020 22:01 SoccerdudeDylan
A solution of 6.85g of a carbohydrate in 100.0g of water has a density of 1.024 g/mL and an osmotic pressure of 4.61 atm at 200C. Calculate molar mass of the carbohydrate

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which term describes a fracture in the earth at which land stays in the same place? a. joint b. fault c. split d. hinge
Answers: 1

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3
You know the right answer?
A solution of 6.85g of a carbohydrate in 100.0g of water has a density of 1.024 g/mL and an osmotic...
Questions

English, 21.05.2021 21:10

Mathematics, 21.05.2021 21:10

Mathematics, 21.05.2021 21:10

Mathematics, 21.05.2021 21:10




Mathematics, 21.05.2021 21:10

English, 21.05.2021 21:10


Chemistry, 21.05.2021 21:10

Mathematics, 21.05.2021 21:10


Mathematics, 21.05.2021 21:10



Mathematics, 21.05.2021 21:10