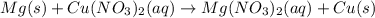When a strip of magnesium metal is placed in an aqueous solution of copper(II) nitrate, elemental copper coats the surface of the magnesium strip and aqueous magnesium nitrate forms.
1. As a reactant, what is the charge on copper?
2. As a product, what is the charge on copper?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
When a strip of magnesium metal is placed in an aqueous solution of copper(II) nitrate, elemental co...
Questions


Mathematics, 24.04.2020 05:57


Mathematics, 24.04.2020 05:57

Mathematics, 24.04.2020 05:57

English, 24.04.2020 05:57



Mathematics, 24.04.2020 05:57

Mathematics, 24.04.2020 05:57



Physics, 24.04.2020 05:57


Mathematics, 24.04.2020 05:57

Mathematics, 24.04.2020 05:57

Business, 24.04.2020 05:57



Mathematics, 24.04.2020 05:57

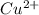 ).
).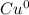 ).
).