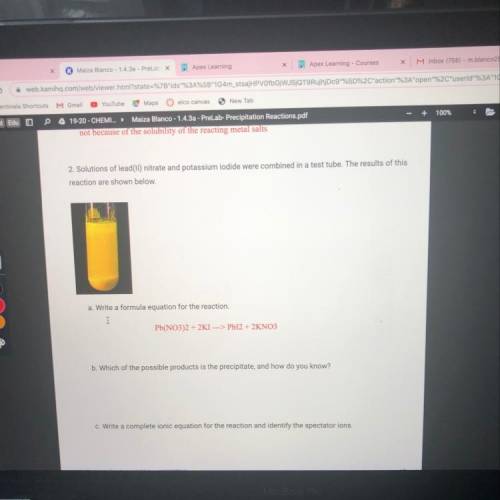
C. Write a complete ionic equation for the reaction and identify the spectator ions.
...

Chemistry, 15.07.2020 20:01 leriscepowell9004
C. Write a complete ionic equation for the reaction and identify the spectator ions.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1

You know the right answer?
Questions

Mathematics, 28.01.2021 18:20

Biology, 28.01.2021 18:20

Mathematics, 28.01.2021 18:20

Mathematics, 28.01.2021 18:20

Mathematics, 28.01.2021 18:20

Biology, 28.01.2021 18:20

Social Studies, 28.01.2021 18:20


Mathematics, 28.01.2021 18:20

History, 28.01.2021 18:20


Mathematics, 28.01.2021 18:20


English, 28.01.2021 18:20

History, 28.01.2021 18:20

History, 28.01.2021 18:20



History, 28.01.2021 18:20

Mathematics, 28.01.2021 18:20



