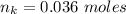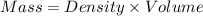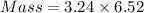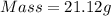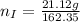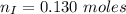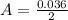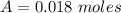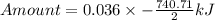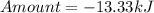Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2
You know the right answer?
How much heat is liberated at constant pressure when 1.41 g of potassium metal reacts with 6.52 mL o...
Questions

Mathematics, 18.11.2020 19:20


Mathematics, 18.11.2020 19:20


Mathematics, 18.11.2020 19:20

Mathematics, 18.11.2020 19:20

Mathematics, 18.11.2020 19:20

Advanced Placement (AP), 18.11.2020 19:20



Physics, 18.11.2020 19:20




Mathematics, 18.11.2020 19:20

Mathematics, 18.11.2020 19:20

Mathematics, 18.11.2020 19:20



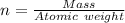
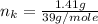 ---where 39 is the atomic weight of potassium
---where 39 is the atomic weight of potassium