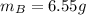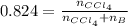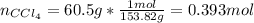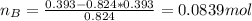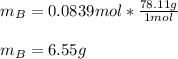Chemistry, 16.07.2020 01:01 jennamcasey94
A solution was made by mixing benzene () and carbon tetrachloride (). Given that the mole fraction of carbon tetrachloride is 0.824 in the solution obtained from 60.5 g , calculate the mass of benzene used. Mass

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Acetic acid, hc2h3o2, dissolves and completely dissociates in water and a solvation sphere of water molecules forms around the ions. this solute-solvent interaction
Answers: 1


Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1
You know the right answer?
A solution was made by mixing benzene () and carbon tetrachloride (). Given that the mole fraction o...
Questions

Chemistry, 12.02.2021 03:10

Mathematics, 12.02.2021 03:10

Mathematics, 12.02.2021 03:10

Physics, 12.02.2021 03:10


History, 12.02.2021 03:10

Mathematics, 12.02.2021 03:10

Mathematics, 12.02.2021 03:20



Physics, 12.02.2021 03:20



Mathematics, 12.02.2021 03:20

Mathematics, 12.02.2021 03:20

Mathematics, 12.02.2021 03:20

Mathematics, 12.02.2021 03:20

Geography, 12.02.2021 03:20


Social Studies, 12.02.2021 03:20