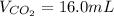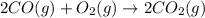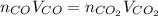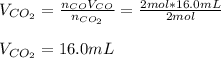Chemistry, 15.07.2020 09:01 westlakebuddy1229
Assuming the same temperature and pressure for each gas, how many milliliters of carbon dioxide are produced from 16 0 mL of CO
2 CO(g) + O2(g) 4, 2 CO2 (g)
Express your answer with the appropriate units.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1


Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2
You know the right answer?
Assuming the same temperature and pressure for each gas, how many milliliters of carbon dioxide are...
Questions


Biology, 20.04.2020 20:11

English, 20.04.2020 20:11

Computers and Technology, 20.04.2020 20:11




Mathematics, 20.04.2020 20:11




Chemistry, 20.04.2020 20:11


Chemistry, 20.04.2020 20:11

English, 20.04.2020 20:11


Mathematics, 20.04.2020 20:11

Social Studies, 20.04.2020 20:11