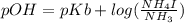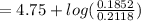Chemistry, 15.07.2020 02:01 kevinvalencia01
An aqueous solution contains 0.397 M ammonia. Calculate the pH of the solution after the addition of 4.63 x 10-2 moles of perchloric acid (HClO4) to 250 mL of this solution. (Assume the volume does not change upon adding perchloric acid). Ka = 5.7 x 10-10, Kb = 1.80 x 10-5

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2
You know the right answer?
An aqueous solution contains 0.397 M ammonia. Calculate the pH of the solution after the addition of...
Questions


Computers and Technology, 04.11.2020 08:00

History, 04.11.2020 08:00

Advanced Placement (AP), 04.11.2020 08:00




Mathematics, 04.11.2020 08:00





History, 04.11.2020 08:00

Mathematics, 04.11.2020 08:00

Biology, 04.11.2020 08:00



Advanced Placement (AP), 04.11.2020 08:00

English, 04.11.2020 08:00

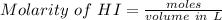
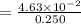
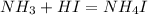
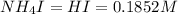
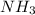 left is
left is