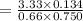When H2O and CO react at 979°C, the products are CO2 and H2. The equilibrium constant (in terms of equilibrium concentrations of reactants and products) for the reaction below is 0.66 at 979°C. If the following concentrations are measured after the reaction reaches equilibrium, what is the concentration of CO(g) in the equilibrated mixture? answer will be in M
Component: Measured Equilibrium Concentration
A. H2 0 (g) 0.750 M
B. CO2 (g) 0.134 M
C. H2 (g) 3.33 M

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1


Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
When H2O and CO react at 979°C, the products are CO2 and H2. The equilibrium constant (in terms of e...
Questions


Physics, 12.02.2021 19:20

Mathematics, 12.02.2021 19:20

Mathematics, 12.02.2021 19:20

Computers and Technology, 12.02.2021 19:20

Mathematics, 12.02.2021 19:20

Mathematics, 12.02.2021 19:20

Mathematics, 12.02.2021 19:20

Mathematics, 12.02.2021 19:20

Mathematics, 12.02.2021 19:20

Mathematics, 12.02.2021 19:20

Mathematics, 12.02.2021 19:20




Mathematics, 12.02.2021 19:20

Biology, 12.02.2021 19:20

Mathematics, 12.02.2021 19:20


Mathematics, 12.02.2021 19:20

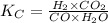
 = equilibrium constant
= equilibrium constant