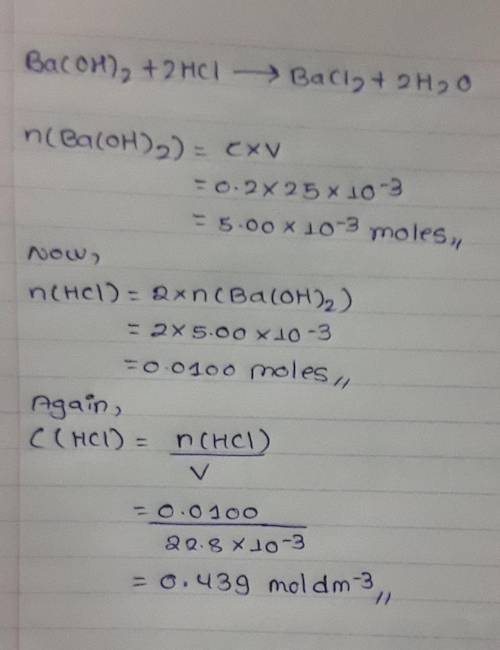Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Which form of relativism states that people rely on their own standards of right and wrong when making a decision?
Answers: 1

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

You know the right answer?
25cm3 of 0.2mol/dm3 barium hydroxide solution reacted with 22.8cm3 hydrochloric acid. Calculate the...
Questions



History, 07.10.2020 14:01




English, 07.10.2020 14:01


SAT, 07.10.2020 14:01

Biology, 07.10.2020 14:01



Mathematics, 07.10.2020 14:01

Mathematics, 07.10.2020 14:01




Computers and Technology, 07.10.2020 14:01


Biology, 07.10.2020 14:01