
Chemistry, 14.07.2020 01:01 Prettygirlyaya
The equilibrium constant in terms of pressures for the reaction COBr2(g) CO(g) + Br2(g) is Kp = 5.40 at 346 K. (a) A pure sample of gaseous carbonyl bromide, COBr2(g), is introduced into a rigid flask at a temperature of 346 K so that its original pressure is 0.123 atm. Calculate the fraction of this starting material that is converted to products at equilibrium.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which term best describes the form sound takes as it travels away from a drum (a- gas)(b-music) ( c-waves) (d-particles
Answers: 3

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2
You know the right answer?
The equilibrium constant in terms of pressures for the reaction COBr2(g) CO(g) + Br2(g) is Kp = 5.40...
Questions

Mathematics, 14.01.2020 00:31


Mathematics, 14.01.2020 00:31

Physics, 14.01.2020 00:31

Mathematics, 14.01.2020 00:31




Mathematics, 14.01.2020 00:31



Mathematics, 14.01.2020 00:31





History, 14.01.2020 00:31


Health, 14.01.2020 00:31

Computers and Technology, 14.01.2020 00:31

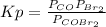 = 5.40
= 5.40

