
Chemistry, 14.07.2020 01:01 laurachealsy923
Calculate Ecell at 80 ºC for a voltaic cell based on the following redox reaction: H2(g, 1.25 atm) + 2AgCl(s) → 2Ag(s) + 2H+(aq, 0.10 M) + 2Cl–(aq, 1.5 M) The standard cell potential Eºcell = +0.18 V at this temperature.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3


Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1
You know the right answer?
Calculate Ecell at 80 ºC for a voltaic cell based on the following redox reaction: H2(g, 1.25 atm) +...
Questions

English, 04.04.2020 19:58

Biology, 04.04.2020 19:58


Mathematics, 04.04.2020 19:59




Geography, 04.04.2020 19:59

SAT, 04.04.2020 20:00










Biology, 04.04.2020 20:00

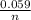 logQ
logQ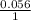 log 0.067
log 0.067


