
Chemistry, 14.07.2020 02:01 lucasrandall
g To what volume (in mL) should 5.07 mL of an 6.82 M acetic acid solution be diluted in order to obtain a final solution that is 0.49 M

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2


Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 23.06.2019 02:00
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1
You know the right answer?
g To what volume (in mL) should 5.07 mL of an 6.82 M acetic acid solution be diluted in order to obt...
Questions

Mathematics, 02.03.2021 19:10

Mathematics, 02.03.2021 19:10

Mathematics, 02.03.2021 19:10

Chemistry, 02.03.2021 19:10

Mathematics, 02.03.2021 19:10

Mathematics, 02.03.2021 19:10

Mathematics, 02.03.2021 19:10


Health, 02.03.2021 19:10


Mathematics, 02.03.2021 19:10

History, 02.03.2021 19:10

Mathematics, 02.03.2021 19:10

Mathematics, 02.03.2021 19:10



Mathematics, 02.03.2021 19:10

Mathematics, 02.03.2021 19:10

Spanish, 02.03.2021 19:10

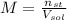
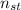 - Amount of moles of solute, measured in moles.
- Amount of moles of solute, measured in moles.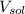 - Volume of the solution, measured in liters.
- Volume of the solution, measured in liters.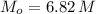 and
and 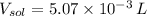 ):
):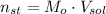
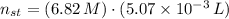
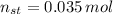
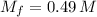 and
and 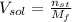
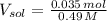
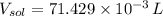
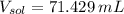 (1 L = 1000 mL)
(1 L = 1000 mL)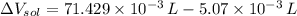
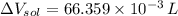
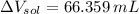 (1 L = 1000 mL)
(1 L = 1000 mL)

