
Chemistry, 14.07.2020 01:01 eggemotions
The molar mass of H2O is 18.01 g/mol. The molar mass of O2 is 32.00 g/mol. What mass of H2O, ins grams, must react to produce 50.00 g of O2
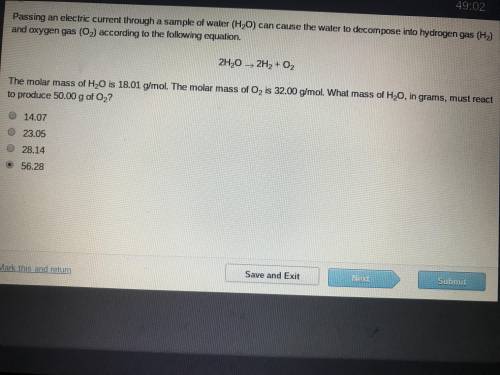

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
You know the right answer?
The molar mass of H2O is 18.01 g/mol. The molar mass of O2 is 32.00 g/mol. What mass of H2O, ins gra...
Questions

Mathematics, 08.07.2019 01:00

Mathematics, 08.07.2019 01:00



Mathematics, 08.07.2019 01:00


Mathematics, 08.07.2019 01:00

Mathematics, 08.07.2019 01:00

Mathematics, 08.07.2019 01:00



English, 08.07.2019 01:00

Physics, 08.07.2019 01:00

History, 08.07.2019 01:00

Mathematics, 08.07.2019 01:00

Health, 08.07.2019 01:00




Mathematics, 08.07.2019 01:00



