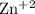Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 00:00
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1
You know the right answer?
Study the following equation and in each case decide whether the substance in bold type has been oxi...
Questions


Advanced Placement (AP), 31.07.2019 10:30






Mathematics, 31.07.2019 10:30

History, 31.07.2019 10:30





Mathematics, 31.07.2019 10:30

English, 31.07.2019 10:30



Computers and Technology, 31.07.2019 10:30


Mathematics, 31.07.2019 10:30