

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:40
Enough of a monoprotic weak acid is dissolved in water to produce a 0.0172 m solution. if the ph of the resulting solution is 2.39 at 20 °c, determine the pka for the acid.
Answers: 1

Chemistry, 21.06.2019 15:10
Seawater contains approximately 3.5%nacl by mass and has a density of 1.02 g/ml. what volume of seawater contains 7.5 g of sodium?
Answers: 2

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1
You know the right answer?
Question 3 (11 points)
A gas has a volume of 690.0mL at -15.1°C and 392.0 mmHg. What would the volu...
Questions


Mathematics, 24.02.2021 05:30

History, 24.02.2021 05:30





Mathematics, 24.02.2021 05:30

Computers and Technology, 24.02.2021 05:30







English, 24.02.2021 05:30


Social Studies, 24.02.2021 05:30


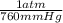 = 0.516 atm
= 0.516 atm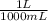 = 0.69 L
= 0.69 L


