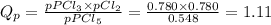Chemistry, 13.07.2020 21:01 ROBIOX5934
At T = 250 °C the reaction PCl5(g) PCl3(g) + Cl2(g) has an equilibrium constant in terms of pressures Kp = 2.15. (a) Suppose the initial partial pressure of PCl5 is 0.548 atm, and PPCl3 = PCl2 = 0.780 atm. Calculate the reaction quotient Qp and state whether the reaction proceeds to the right or to the left as equilibrium is approached

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2
You know the right answer?
At T = 250 °C the reaction PCl5(g) PCl3(g) + Cl2(g) has an equilibrium constant in terms of pressure...
Questions


Mathematics, 18.04.2021 20:50



Mathematics, 18.04.2021 20:50

Biology, 18.04.2021 20:50

English, 18.04.2021 20:50

Social Studies, 18.04.2021 20:50

Mathematics, 18.04.2021 20:50


Mathematics, 18.04.2021 20:50

Mathematics, 18.04.2021 20:50


Mathematics, 18.04.2021 20:50



Mathematics, 18.04.2021 20:50

Mathematics, 18.04.2021 20:50

Health, 18.04.2021 20:50