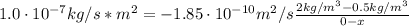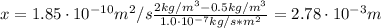A sheet of steel 2.5-mm thick has nitrogen atmospheres on both sides at 900"C and is permitted to achieve a steady-state diffusion condition. The diffusion coefficient for nitrogen in steel at this temperature is 1.85 x 10^-10 m^2/s, and the diffusion flux is found to be 1.0 x 10^-7 kg/m^2 s. Also, it is known that the concentration of nitrogen in the steel at the high-pressure surface is 2 kg/m^3. How far into the sheet from this high-pressure side will the concentration be 0.5 kg/m3? Assume a linear concentration profile.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Agroup of students is studying convection currents. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other in an area with warm air. after 10 minutes, the balloons are released from a height of 1 meter. which of the following do the students most likely observe? a. the balloons both rise. the cold balloon is larger than the warm balloon. b. the balloons rise at the same rate. both balloons are the same size. c. the warm balloon expands and rises. the cold balloon shrinks and sinks. d. the cold balloon expands and rises. the warm balloon shrinks and sinks.
Answers: 2

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

You know the right answer?
A sheet of steel 2.5-mm thick has nitrogen atmospheres on both sides at 900"C and is permitted to ac...
Questions




English, 18.02.2020 18:36

Computers and Technology, 18.02.2020 18:36



Health, 18.02.2020 18:36








English, 18.02.2020 18:36

Mathematics, 18.02.2020 18:37



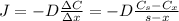
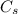 : is the nitrogen concentration in the surface of steel = 2 kg/m³
: is the nitrogen concentration in the surface of steel = 2 kg/m³ 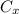 : is the nitrogen concentration in the point x = 0.5 kg/m³
: is the nitrogen concentration in the point x = 0.5 kg/m³