Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1

Chemistry, 23.06.2019 07:00
In order for a high temperature boiler or steam engine to produce superheated water, or steam: the heat source must be greater than 100°c the water must be permitted to evaporate quickly the system must be sealed and become pressurized above atmospheric pressure the vapor pressure must be kept below 760 mm(hg)
Answers: 1
You know the right answer?
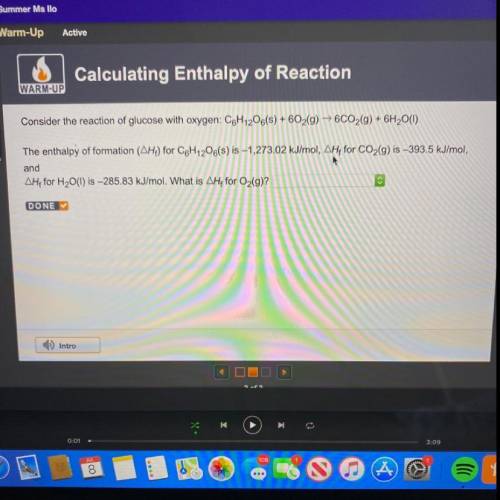
Consider the reaction of glucose with oxygen: C6H12O6(s) + 602(g) → 6CO2(g) + 6H2O(1)
The enthalpy...
Questions



Geography, 27.12.2020 08:10




Mathematics, 27.12.2020 08:10

English, 27.12.2020 08:10

History, 27.12.2020 08:10

Arts, 27.12.2020 08:10



Computers and Technology, 27.12.2020 08:10

Geography, 27.12.2020 08:10

Arts, 27.12.2020 08:10


Mathematics, 27.12.2020 08:10


Mathematics, 27.12.2020 08:10

Mathematics, 27.12.2020 08:10