Chemistry, 09.07.2020 14:01 aliyahadekoya
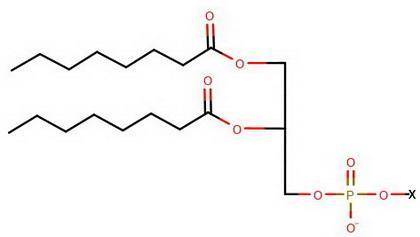
A certain phospholipid molecule contains two fatty acid chains of eight carbon atoms each having no carbon-carbon double bonds in the chain. The phosphate ion is attached to a polar
organic molecule, X, just as highlighted in yellow in the figure in the introduction.
Complete the structure of the phospholipid molecule using the structure in the introduction. To include a X group in your structure, draw another atom, hover over the atom with the
mouse, and then press the X key on your keyboard.
Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1


Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
A certain phospholipid molecule contains two fatty acid chains of eight carbon atoms each having no...
Questions

Mathematics, 29.08.2019 16:30

Physics, 29.08.2019 16:30

Mathematics, 29.08.2019 16:30


Mathematics, 29.08.2019 16:30


Biology, 29.08.2019 16:30


History, 29.08.2019 16:30

Geography, 29.08.2019 16:30


Mathematics, 29.08.2019 16:30

Mathematics, 29.08.2019 16:30

Mathematics, 29.08.2019 16:30

Advanced Placement (AP), 29.08.2019 16:30





Biology, 29.08.2019 16:30