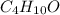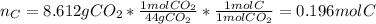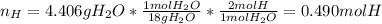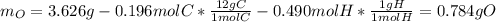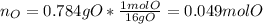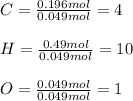Chemistry, 09.07.2020 02:01 germainenez3288
The compound known as diethyl ether, commonly referred to as ether, contains carbon, hydrogen, and oxygen. A 3.626 g sample of ether was combusted in an oxygen rich environment to produce 8.612 g of CO2(g) and 4.406 g of H2O(g). Insert subscripts to complete the empirical formula of ether.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2
You know the right answer?
The compound known as diethyl ether, commonly referred to as ether, contains carbon, hydrogen, and o...
Questions

Biology, 27.01.2020 21:31






Geography, 27.01.2020 21:31

Business, 27.01.2020 21:31


Health, 27.01.2020 21:31



History, 27.01.2020 21:31

English, 27.01.2020 21:31

Mathematics, 27.01.2020 21:31

History, 27.01.2020 21:31



Computers and Technology, 27.01.2020 21:31

Mathematics, 27.01.2020 21:31