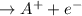Chemistry, 09.07.2020 01:01 gibbss80stu
In some chemical reactions, electrons are transferred from one substance to another, but there is no net gain or loss of electrons during the reaction. These reactions involve the oxidation, or loss of electrons, of one substance, and the reduction, or gain of electrons, of another. This type of reaction is called a redox reaction. In neutral atoms, such as A and X, the number of protons equals the number of electrons. When atom A loses an electron, the positive charge of the nucleus becomes greater than the negative charge of the electrons, and the resulting ion has a net positive charge, forming a cation. In a reaction, the electron must be taken up by another atom, X , which results in more electrons than protons and a net negative charge on the atom, forming an anion. Mnemonic devices can help in remembering the definitions of oxidation and reduction. Two common mnemonic devices are LEO (Losing Electrons: Oxidation) says GER (Gaining Electrons: Reduction), and OIL (Oxidation Is Losing) RIG (Reduction Is Gaining). Identify the equations that show the oxidation of neutral atom A and the reduction of neutral a

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1


You know the right answer?
In some chemical reactions, electrons are transferred from one substance to another, but there is no...
Questions

English, 21.01.2021 19:10


Mathematics, 21.01.2021 19:10

Health, 21.01.2021 19:10

Biology, 21.01.2021 19:10

Mathematics, 21.01.2021 19:10

Biology, 21.01.2021 19:10



Mathematics, 21.01.2021 19:10

English, 21.01.2021 19:10


English, 21.01.2021 19:10

Mathematics, 21.01.2021 19:10




Mathematics, 21.01.2021 19:10


English, 21.01.2021 19:10