Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which is a character of nuclear fusion but not nuclear fission
Answers: 3

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 23.06.2019 01:00
How does carbon monoxide pose the greatest threat to humans? a. it can be produced by wood fires. b. it can be produced by home furnaces. c. it is produced by acid rain. d. it is produced by modern automobiles.
Answers: 2

Chemistry, 23.06.2019 06:20
Examine the false statement. compounds are the smallest unit of an element that occur most commonly in nature. select the rewording of the statement that is true. a: atoms are the smallest unit of an element that commonly occur in nature. b: molecules are the smallest unit of an element or compound that commonly occur in nature. c: molecules are the smallest unit of a compound that occur on the periodic table. d: compounds are the smallest unit of an element that occur on the periodic table
Answers: 1
You know the right answer?
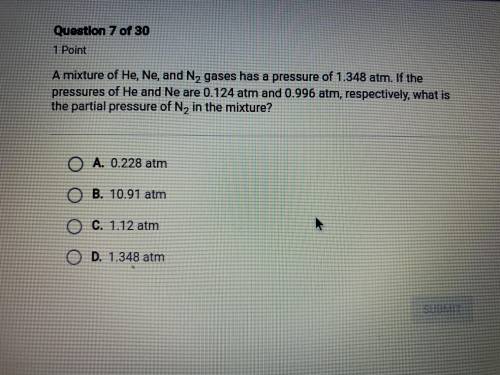
a mixture of He, Ne, and N2 gases has a pressure of 1.348 atm. if the pressures of He and Ne are 0.1...
Questions

History, 18.12.2019 06:31

Mathematics, 18.12.2019 06:31




Mathematics, 18.12.2019 06:31



Mathematics, 18.12.2019 06:31



History, 18.12.2019 06:31

English, 18.12.2019 06:31



History, 18.12.2019 06:31


Biology, 18.12.2019 06:31