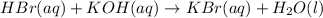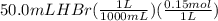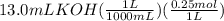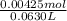Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3
You know the right answer?
A50.0ml- volume of 0.15m hbr is titrated with 0.25m koh . calculate the ph after the addition of 13....
Questions




Mathematics, 23.09.2019 07:30


Physics, 23.09.2019 07:30

Biology, 23.09.2019 07:30


Health, 23.09.2019 07:30

Mathematics, 23.09.2019 07:30

English, 23.09.2019 07:30

Health, 23.09.2019 07:30






History, 23.09.2019 07:30

Biology, 23.09.2019 07:30

Mathematics, 23.09.2019 07:30