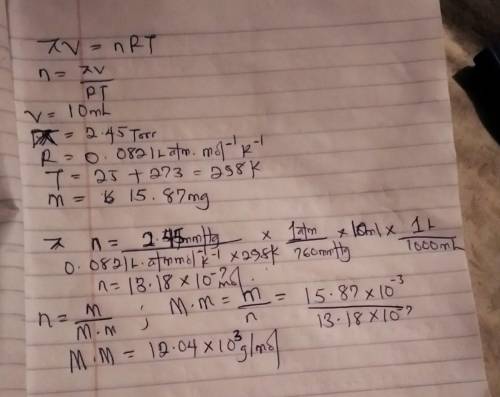Chemistry, 06.07.2020 09:01 junielouwho
The osmotic pressure of a solution containing 15.87 mg of an unknown protein per 10.0 mL of solution is 2.45 torr at 25oC. Find the molar mass of the unknown protein.

Answers: 3


Another question on Chemistry



Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 3

You know the right answer?
The osmotic pressure of a solution containing 15.87 mg of an unknown protein per 10.0 mL of solution...
Questions

Computers and Technology, 25.09.2021 15:50

History, 25.09.2021 15:50

English, 25.09.2021 15:50


English, 25.09.2021 15:50

Geography, 25.09.2021 15:50



English, 25.09.2021 15:50



Mathematics, 25.09.2021 15:50

History, 25.09.2021 15:50


Mathematics, 25.09.2021 15:50

Mathematics, 25.09.2021 15:50


English, 25.09.2021 15:50

Mathematics, 25.09.2021 15:50