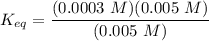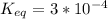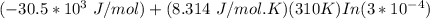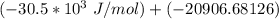Chemistry, 05.07.2020 14:01 lilrariwmb23701
A critical reaction in the production of energy to do work or drive chemical reactions in biological systems is the hydrolysis of adenosine triphosphate, ATP, to adenosine diphosphate, ADP, as described by the reactionATP(aq)+ H2O(l) → ADP(aq)+ HPO4^-2 (aq)for which ΔGrxn = -30.5 kj/mol at 37.0C and pH 7.0. Required:a. Calculate the value of ΔGrxn in a biological cell in which [ATP] = 5.0 mM, [ADP] = 0.30 mM, and HPO4^-2= 5.0mMb. Is the hydrolysis of ATP spontaneous under these conditions?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1


Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
You know the right answer?
A critical reaction in the production of energy to do work or drive chemical reactions in biological...
Questions

Social Studies, 29.01.2021 20:00


Mathematics, 29.01.2021 20:00

Spanish, 29.01.2021 20:00




History, 29.01.2021 20:00


Spanish, 29.01.2021 20:00

Mathematics, 29.01.2021 20:00

Mathematics, 29.01.2021 20:00

Mathematics, 29.01.2021 20:00

History, 29.01.2021 20:00




Mathematics, 29.01.2021 20:10


Spanish, 29.01.2021 20:10

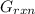 = −51. 4 kJ/mol
= −51. 4 kJ/mol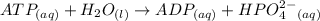
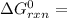 -30.5 kJ/mol
-30.5 kJ/mol![[HPO_4^{2-}}]](/tpl/images/0701/7595/d50c3.png) = 5.0 mM
= 5.0 mM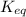 can be expressed as:
can be expressed as:![K_{eq} = \dfrac{[ADP][ HPO_4^{2-}]} {[ATP]}](/tpl/images/0701/7595/e3711.png)