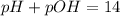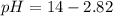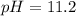Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Asolution has a ca2+ concentration of 0.049 m and an f- concentration is 0.147 m at equilibrium. the value of ksp for caf2 at 25°c is 4.0 x 10-11. will this solution form a precipitate? yes no
Answers: 3

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2
You know the right answer?
A chemist dissolves 867. mg of pure barium hydroxide in enough water to make up 170. mL of solution....
Questions




Mathematics, 23.02.2021 18:10

Mathematics, 23.02.2021 18:10

Mathematics, 23.02.2021 18:10

Mathematics, 23.02.2021 18:10








Social Studies, 23.02.2021 18:10

Mathematics, 23.02.2021 18:10

History, 23.02.2021 18:10



Mathematics, 23.02.2021 18:10

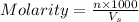
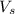 = volume of solution in ml
= volume of solution in ml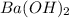 =
= 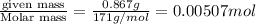 (1g=1000mg)
(1g=1000mg)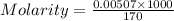
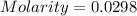
![pOH=-\log [OH^-]](/tpl/images/0701/6467/1fac1.png)
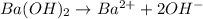
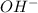
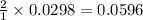 moles of
moles of ![pOH=-\log[0.0596]=2.82](/tpl/images/0701/6467/015aa.png)