Chemistry, 05.07.2020 14:01 jeffylovesgreenbeans
Two evacuated bulbs of equal volume are connected by a tube of negligible volume. One of the bulbs is placed in a constant-temperature bath at 245 K and the other bulb is placed in a constant-temperature bath at 350 K . Exactly 5 mol of an ideal gas is injected into the system. Calculate the final number of moles of gas in each bulb.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
You know the right answer?
Two evacuated bulbs of equal volume are connected by a tube of negligible volume. One of the bulbs i...
Questions

History, 02.08.2019 19:00



















Physics, 02.08.2019 19:00

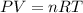 (1)
(1)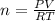
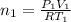
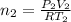
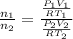
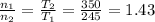
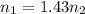 (2)
(2)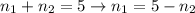 (3)
(3)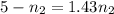
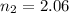 (4)
(4)