
Chemistry, 05.07.2020 14:01 FailingstudentXD
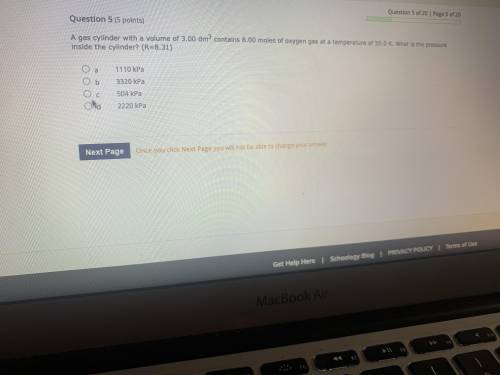
Because cylinder with a volume of 3.00dm^3 contains 8.00 moles of oxygen gas at a temperature of 50.0 K. What is the pressure inside the cylinder? ( R=8.31)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2

You know the right answer?
Because cylinder with a volume of 3.00dm^3 contains 8.00 moles of oxygen gas at a temperature of 50....
Questions

Mathematics, 06.09.2019 07:10

Physics, 06.09.2019 07:10

Biology, 06.09.2019 07:10


History, 06.09.2019 07:10



Mathematics, 06.09.2019 07:10

Biology, 06.09.2019 07:10

History, 06.09.2019 07:10

Mathematics, 06.09.2019 07:10

English, 06.09.2019 07:10


Mathematics, 06.09.2019 07:10


History, 06.09.2019 07:10


Mathematics, 06.09.2019 07:10




