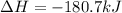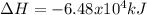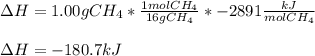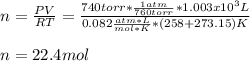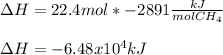Chemistry, 04.07.2020 02:01 khikhi1705
Consider the following reaction:
CH4 +2O2 → CO2 + 2H2O. ΔH= -2891 kJ
Calculate the enthalpy change for each of the following cases:
a. 1.00 g methane is burned in excess oxygen.
b. 1.00 3 10^3 L methane gas at 740. torr and 258°C are burned in excess oxygen.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 23.06.2019 03:30
How many grams of sodium chloride are in 250ml of a 2.5m naci solution
Answers: 1
You know the right answer?
Consider the following reaction:
CH4 +2O2 → CO2 + 2H2O. ΔH= -2891 kJ
Calculate the enth...
Calculate the enth...
Questions




Mathematics, 26.07.2019 20:30




Advanced Placement (AP), 26.07.2019 20:30