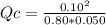The reaction of nitrogen gas and oxygen gas to form nitrogen monoxide gas is shown below. If the measured concentrations of all three chemicals at some point in time are: [N2] = 0.80 M
[O2] = 0.050 M
[NO] = 0.10 M
Which statement is TRUE about the reaction at this point in time? N2(g) + O2(g) ⇄ 2 NO(g) K = 0.10
The reaction is at equilibrium.
The reverse reaction is occurring at a faster rate than the forward reaction.
The forward reaction is occurring at a faster rate than the reverse reaction.
This set of concentration values is impossible because the concentrations of N2 and O2 must be the same.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3
You know the right answer?
The reaction of nitrogen gas and oxygen gas to form nitrogen monoxide gas is shown below. If the mea...
Questions




Mathematics, 31.03.2021 05:50





Mathematics, 31.03.2021 05:50




English, 31.03.2021 05:50

Mathematics, 31.03.2021 05:50


Mathematics, 31.03.2021 05:50


Mathematics, 31.03.2021 05:50


![Qc=\frac{[C]^{c}*[D]^{d} }{[A]^{a}*[B]^{b} }](/tpl/images/0700/8667/038b9.png)
![Qc=\frac{[NO]^{2} }{[N_{2} ]*[O_{2} ] }](/tpl/images/0700/8667/47464.png)