Chemistry, 03.07.2020 20:01 kwoolfe59006
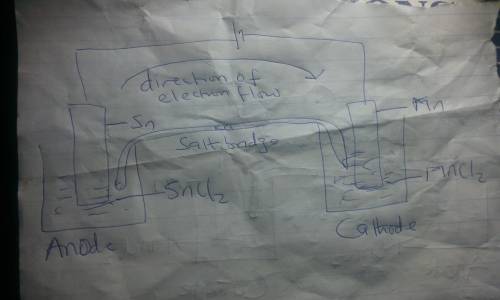
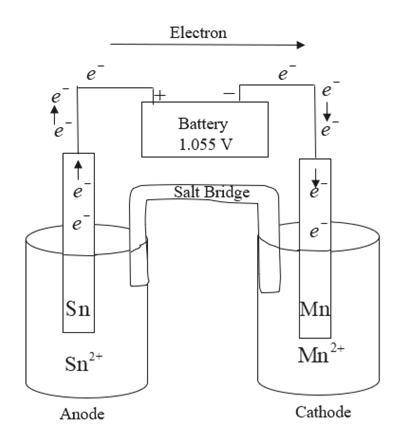
Draw an electrolytic cell in which Mn2+ is reduced to Mn and Sn is oxidized to Sn2+. (Assume standard conditions).
Part A Label the anode and cathode, indicate the direction of electron flow.
Drag the appropriate labels to their respective targets.
Part B Write an equation for the half-reaction occurring at each electrode.
Express your answers as chemical equations separated by a comma. Identify all of the phases in your answer.
Part C What minimum voltage is necessary to drive the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
Draw an electrolytic cell in which Mn2+ is reduced to Mn and Sn is oxidized to Sn2+. (Assume standar...
Questions

Mathematics, 04.10.2019 19:00


Mathematics, 04.10.2019 19:00

Computers and Technology, 04.10.2019 19:00





Business, 04.10.2019 19:00


Geography, 04.10.2019 19:00



Mathematics, 04.10.2019 19:00

English, 04.10.2019 19:00




History, 04.10.2019 19:00